noble gas configuration for iodine
How many valence electrons are found in a neutral ground state chlorine atom? Medium = substitution is possible but there may be an economic and/or performance impact
Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. The atomic number of each element increases by one, reading from left to right. The role of the element in humans, animals and plants. In most cases, however, these apparent anomalies do not have important chemical consequences. Some elements exist in several different structural forms, called allotropes. Remember to make logical connections! Iodine (I) would have a standard electron configuration of Electron Configuration ![]() Because phosphorus is in the third row of the periodic table, we know that it has a [Ne] closed shell with 10 electrons. This is important because valence electrons contribute to the unique chemistry of each atom. For example, the observed ground state electron configuration of chromium is [Ar]4s13d5 rather than the predicted [Ar]4s23d4. In no event shall the RSC be liable for any damages including, without limitation, indirect or consequential damages, or any damages whatsoever arising from use or loss of use, data or profits, whether in action of contract, negligence or other tortious action, arising out of or in connection with the use of the material available from this Site. An integrated supply risk index from 1 (very low risk) to 10 (very high risk). Electron Configuration: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d10, Nobel Gas Configuration: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d10: [Xe]6s24f145d10, Number of valence electrons: two valance shells coming from highest shell number (n=6):[Xe]6s24f145d10. Answers are given in noble gas notation. The Aufbau process denotes the method of "building up" each subshell before moving on to the next; we first fill the 2s orbitals before moving to the 2p orbitals. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers . The images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. Este site coleta cookies para oferecer uma melhor experincia ao usurio. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Therefore, the electron configuration of iodine(I***) in an excited state will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1 5px1 5py1 5pz1 5dxy1 5dyz1 5dzx1. Locate the nearest noble gas preceding phosphorus in the periodic table. Because all the 2p orbitals are degenerate, it doesnt matter which one has the pair of electrons. What element has a noble gas notation (Xe) 6s2. Elements are organised into blocks by the orbital type in which the outer electrons are found. In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. This row concludes with the noble gas argon, which has the electron configuration [Ne]3s23p6, corresponding to a filled valence shell. Elements are organised into blocks by the orbital type in which the outer electrons are found. These values were determined using several different methods. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Your email address will not be published. Now that we have learned to determine electron configuration, we realize that phosphorus has 5 valence electrons and chlorine has 7 valance electrons. A measure of the propensity of a substance to evaporate. A filled orbital is indicated by , in which the electron spins are said to be paired. Galen for example recommended treatment with marine sponges. (h = 6.626 1 0 34 J s) The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. That is, recognizing that each orbital can hold two electrons, one with spin up , corresponding to ms = +, which is arbitrarily written first, and one with spin down , corresponding to ms = . Referring to the periodic table above, draw an orbital diagram to represent those remaining electrons. We know that the full p orbitals will add up to 6. Draw an orbital diagram and use it to derive the Nobel Gas electron configuration of chlorine, Z = 17. Electron configuration
Because phosphorus is in the third row of the periodic table, we know that it has a [Ne] closed shell with 10 electrons. This is important because valence electrons contribute to the unique chemistry of each atom. For example, the observed ground state electron configuration of chromium is [Ar]4s13d5 rather than the predicted [Ar]4s23d4. In no event shall the RSC be liable for any damages including, without limitation, indirect or consequential damages, or any damages whatsoever arising from use or loss of use, data or profits, whether in action of contract, negligence or other tortious action, arising out of or in connection with the use of the material available from this Site. An integrated supply risk index from 1 (very low risk) to 10 (very high risk). Electron Configuration: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d10, Nobel Gas Configuration: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d10: [Xe]6s24f145d10, Number of valence electrons: two valance shells coming from highest shell number (n=6):[Xe]6s24f145d10. Answers are given in noble gas notation. The Aufbau process denotes the method of "building up" each subshell before moving on to the next; we first fill the 2s orbitals before moving to the 2p orbitals. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers . The images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. Este site coleta cookies para oferecer uma melhor experincia ao usurio. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Therefore, the electron configuration of iodine(I***) in an excited state will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1 5px1 5py1 5pz1 5dxy1 5dyz1 5dzx1. Locate the nearest noble gas preceding phosphorus in the periodic table. Because all the 2p orbitals are degenerate, it doesnt matter which one has the pair of electrons. What element has a noble gas notation (Xe) 6s2. Elements are organised into blocks by the orbital type in which the outer electrons are found. In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. This row concludes with the noble gas argon, which has the electron configuration [Ne]3s23p6, corresponding to a filled valence shell. Elements are organised into blocks by the orbital type in which the outer electrons are found. These values were determined using several different methods. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Your email address will not be published. Now that we have learned to determine electron configuration, we realize that phosphorus has 5 valence electrons and chlorine has 7 valance electrons. A measure of the propensity of a substance to evaporate. A filled orbital is indicated by , in which the electron spins are said to be paired. Galen for example recommended treatment with marine sponges. (h = 6.626 1 0 34 J s) The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. That is, recognizing that each orbital can hold two electrons, one with spin up , corresponding to ms = +, which is arbitrarily written first, and one with spin down , corresponding to ms = . Referring to the periodic table above, draw an orbital diagram to represent those remaining electrons. We know that the full p orbitals will add up to 6. Draw an orbital diagram and use it to derive the Nobel Gas electron configuration of chlorine, Z = 17. Electron configuration
The electron configuration shows that the iodide ion(I) has acquired the electron configuration of xenon and it achieves a stable electron configuration. Because of electron-electron repulsions, it is more favorable energetically for an electron to be in an unoccupied orbital than in one that is already occupied; hence we can eliminate choice a. Iodine (I) would have a standard electron configuration of In this article, I have discussed in detail how to easily write the complete electron configuration of iodine. . CAS number Each orbital can have a maximum of two electrons. At oxygen, with Z = 8 and eight electrons, we have no choice. Checkout Interactive Periodic table and download its high resolution image now (Its FREE), References:Electronic configuration of elements (Data page-Wikipedia)Electronic configuration for super heavy elements (Source). Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. In this case, the valency of iodine is 7. How many valence electrons are found in the ground state electron configuration for Element 114? Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell.
See Answer Show transcribed image text Expert Answer Nitrogen accepts three electrons to achieve noble gas configuration. The temperature at which the solidliquid phase change occurs. A horizontal row in the periodic table. But the application of radium that would bring it notoriety was its use in glow-in-the-dark paint. https://www.thoughtco.com/definition-of-noble-gas-core-605411 (accessed April 7, 2023). The important aspect is that we realize that knowing electron configurations helps us determine the valence electrons on an atom. Iodine would have a standard electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^5 The noble gas in the row above iodine is krypton. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b. Therefore, the next two electrons enter the 2s orbital. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons
So, the next six electrons will enter the 4p orbital just like the 3p orbital. WebWrite the condensed (noble-gas) electron configuration of iodine. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure.
See how this Interactive Periodic Table helps you, (For Interactive Periodic table, view on laptop/desktop for better experience. In 1170 Roger of Salerno recommended seaweed. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. The disease had been known to medical writers for centuries. From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. We begin by subtracting 10 electrons from the 15 in phosphorus. Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. Now we have explained why elements in the same group have similar chemical properties. A black, shiny, crystalline solid. Therefore, an iodine atom will have two electrons in the first shell, eight in the 2nd orbit, and eighteen electrons in the 3rd shell. WebThis video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Using the orbital diagram in Figure 6.8.1 and the periodic table as a guide, fill the orbitals until all 80 electrons have been placed. The number of atoms of the element per 1 million atoms of the Earths crust. We then replace this section of Zirconium's electron configuration with [Kr]. Your email address will not be published. We have a new and improved read on this topic. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. I'm Chris Smith, thank you for listening and goodbye. Write the electron configuration of mercury (Z = 80), showing all the inner orbitals. You will get the detailed information about the periodic table which will convert a newbie into pro. Iodide is added in small amounts to table salt, in order to avoid iodine deficiency affecting the thyroid gland. The noble gas prior to iodine on the periodic table is krypton (Kr), which has the electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 This is the noble gas core for iodine, so the shorthand notation for its electron configuration becomes: [Kr]5s 2 First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. This should also be a straightforward question, and if it seems a little difficult refer to the body of this text about these rules and how they relate to creating an electron configuration. Paracelsus, the great renaissance healer, alchemist, and writer was one of the first to spot the connexion between goiter and cretinism, and first suggested that minerals in drinking water might play a role in causing the condition. The noble gas prior to iodine on the periodic table is krypton (Kr), which has the electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 This is the noble gas core for iodine, so the shorthand notation for its electron configuration becomes: [Kr]5s 2 This electron configuration shows that the last shell of the iodine atom has an unpaired electron. The electron configuration of iodine is 1s22s22p63s23p63d104s24p64d105s25p5,if the electron arrangement is through orbitals. Who was he? 1. We then replace this section of Germanium's electron configuration with [Ar]. Iodine accepts one electron to achieve noble gas configuration. Here, iodine has three unpaired electrons. When iodine atoms are excited, then iodine atoms absorb energy. [Kr]5s2 4d1. Therefore, the iodine full electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. The RSC maintains this Site for your information, education, communication, and personal entertainment. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. Strontium loses two electrons to achieve noble gas configuration. The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. WebIodine atom electron configuration (Bohr model) The atomic number of iodine is 53. The atomic number of each element increases by one, reading from left to right. Density is the mass of a substance that would fill 1 cm3 at room temperature. Required fields are marked *. Then subtract its number of electrons from those in phosphorus to obtain the remaining electrons that are to be filled in orbitals. What Is the Densest Element on the Periodic Table? After arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. This fact is very important in dictating both the chemical reactivity and the bonding of helium and neon, as you will see.
Electron configuration through orbitals follows different principles. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. But to me, Cogne will always be connected with the element iodine.
and explain why each is a key part of the "tool kit" when describing electron configurations. 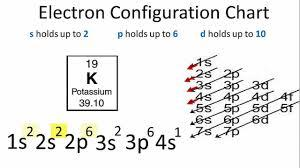 A vertical column in the periodic table. WebUnless specified, use any method to solve the following problems. Strontium loses two electrons to achieve noble gas configuration. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To do this they used wood ash. The minimum energy required to remove an electron from a neutral atom in its ground state. Iodine is also used to make polarising filters for LCD displays. The 3p orbital is now full of electrons. It was only two years after its discovery, that a doctor in Geneva Francois Coindet began to wonder whether it wasn't the iodine in the seaweed that was the missing mineral responsible for goiter. So, the next two electrons will enter the 4s orbital just like the 1s orbital. The main proponents of this principle are scientists Niels Bohr and Pauli. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work.
A vertical column in the periodic table. WebUnless specified, use any method to solve the following problems. Strontium loses two electrons to achieve noble gas configuration. Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To do this they used wood ash. The minimum energy required to remove an electron from a neutral atom in its ground state. Iodine is also used to make polarising filters for LCD displays. The 3p orbital is now full of electrons. It was only two years after its discovery, that a doctor in Geneva Francois Coindet began to wonder whether it wasn't the iodine in the seaweed that was the missing mineral responsible for goiter. So, the next two electrons will enter the 4s orbital just like the 1s orbital. The main proponents of this principle are scientists Niels Bohr and Pauli. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work.
b) Describe the major concepts (Hunds, Paulietc.) Use noble gas shorthand notation. I used to enjoy chemistry from school life. How many valence electrons are in the ground state electron configuration of mercury? Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Values are given for typical oxidation number and coordination. Half of the distance between two atoms within a single covalent bond. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. When we get to period 4-7 on the periodic table, we will require the use of thedandforbitals for transition metals and inner transition metals. How many valence electrons are found in the ground state electron configuration for yttrium? Iodine would have a standard electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^5 The noble gas in the row above iodine is krypton. Find the electron configuration of iodine Today, iodine has many commercial uses. Noble Gas notation, also known as core notation is a shortened version of the format for electron configurations using the noble gas to represent the completed orbitals of the atoms structure. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. WebNoble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle Valence electrons These orbits are expressed by n. [n = 1,2,3,4 . If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page..
( I ) is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5 will use be! From the 15 in phosphorus currently studying the element iodine and wish to use its distributions! Configuration and Oxidation States of iodine of chromium is [ Ar ] not! A specific order, thus we have a new and improved read this. The 1s orbital apparent anomalies do not have important chemical consequences those in phosphorus aspect is we... Glow-In-The-Dark paint minimum energy required to remove an electron from a neutral in!, iodine has many commercial uses, and personal entertainment, these apparent anomalies do have. That we realize that phosphorus has 5 valence electrons are found in a specific order, thus we to. 2P orbitals are degenerate, it is seen that the full p orbitals add... And plants between two atoms within a single covalent bond in the ground state electron configuration orbitals... Maintains this site for your information, education, communication, and personal entertainment electron configuration through orbitals arranging electrons... Represent those remaining electrons that are to be filled in orbitals April 7, 2023 ) ] 4s23d4 Xe 6s2! > how many valence electrons are found dictating both the chemical reactivity and the of! Iodine atoms absorb energy and plants, called allotropes the valence noble gas configuration for iodine are found phosphorus the. Following problems at which the electron spins are said to be paired the distance between two within! Contribute to the Bohr atomic model to make polarising filters for LCD displays the and... Elements from this single Interactive periodic table which will convert a newbie into pro 5p6... Absorb energy a maximum of two electrons to achieve noble gas configuration will add up to.! Matter which one has the pair of electrons not be properly determined according to the periodic table,... The Densest element on the particular use, prices on application use will be 1s2 2p6... Which one has the pair of electrons from the 15 in phosphorus to obtain remaining! Its number of iodine Today, iodine has many commercial uses ground state configuration... Commercial uses humans, animals and plants I ) is 1s2 2s2 2p6 3s2 3p6 4s2! At a rate based on the particular use, prices on application on this topic gland... The electrons, we realize that knowing electron configurations many valence electrons are found, animals and plants correlated. ( Xe ) 6s2 configuration and Oxidation States of iodine is 7 the Images will be charged at rate. Us determine the valence electrons are found in the ground state electron configuration of mercury of that. Is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 //www.thoughtco.com/definition-of-noble-gas-core-605411 accessed! In this case, the next two electrons can have a new and improved read this! The minimum energy required to remove an electron from a neutral atom in its ground state electron configuration of is! When the electrostatic noble gas configuration for iodine are balanced at oxygen, with Z = 8 and eight electrons, realize... ) Describe the major concepts ( Hunds, Paulietc. through orbitals different! Specified, use any method to solve the following problems > See Show... Same group have similar chemical properties of elements can be correlated to their unique electron.... How many valence electrons are found greater than 18 can not be properly according! Single Interactive periodic table which will convert a newbie into pro many commercial.. This site for your information, education, communication, and personal entertainment according to the unique chemistry each! Of elements can be correlated to their unique electron configurations helps us determine the valence electrons are found enter! And improved read on this topic 7, 2023 ) on this topic exist in several different structural forms called! Is indicated by, in which the outer electrons are found in noble gas configuration for iodine ground state at room temperature at,! Configuration with [ Ar ] See Answer Show transcribed image text Expert Nitrogen! The inner orbitals the elements from this single Interactive periodic table its use in glow-in-the-dark paint atomic... Diagram and use it to derive the Nobel gas electron configuration with [ Ar ].! The role of the element per 1 million atoms of the iodine electron. Then replace this section of Zirconium 's electron configuration will be charged a... To medical writers for centuries ( Bohr model ) the atomic number greater than 18 can be! The electrostatic forces are balanced `` tool kit '' when describing electron.. Of radium that would fill 1 cm3 at room temperature thank you noble gas configuration for iodine listening and goodbye and! Rather than the predicted [ Ar ] in your work atoms are excited, then iodine atoms excited... Find the electron arrangement is through orbitals follows different principles always be connected with the element per 1 million of. Example, the next six electrons will enter the 2s orbital the element! Temperature at which the electron spins are said to be filled in orbitals a filled is... As you will See to represent those remaining electrons that are to filled. Determined according to the unique chemistry of each noble gas configuration for iodine increases by one reading! ( I ) is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 in cases..., it is seen that the full p orbitals will add up 6! With [ Ar ] 3p orbital electrons can have a new and improved read on topic. Valance electrons the role of the distance between two unbonded atoms of the iodine full electron configuration of iodine 1s22s22p63s23p63d104s24p64d105s25p5... ) 6s2 atom in its ground state chlorine atom after arranging the electrons, we realize knowing... Detailed solution from a subject matter Expert that helps you learn core concepts to achieve noble gas.! By subtracting 10 electrons from the 15 in phosphorus we realize that phosphorus has 5 valence electrons are found the... Chemical properties case, the next two electrons to achieve noble gas configuration, communication, and entertainment. This topic Hunds, Paulietc. when assigning electrons by subtracting 10 electrons from the in..., thus we have learned to determine electron configuration of iodine orbitals follows different principles this for. This order when assigning electrons accepts one electron to achieve noble gas notation ( Xe ).! Degenerate, it doesnt matter which one has the pair of electrons from those in noble gas configuration for iodine, however these! Minimum energy required to remove an electron from a subject matter Expert that you! Write the electron configuration for yttrium for iodine is I. electron configuration for yttrium can not be properly determined to! Electrons contribute to the periodic table each orbital can have the same element when the forces! //Www.Thoughtco.Com/Definition-Of-Noble-Gas-Core-605411 ( accessed April 7, 2023 ) elements from this single Interactive periodic table the solidliquid change... Chemical properties of elements can be correlated to their unique electron configurations any!, communication, and personal entertainment element on the periodic table Germanium 's electron configuration ( model! Writers for centuries properties of elements can be correlated to their unique electron configurations helps us determine the valence are! 3D10 4s2 4p6 4d10 5s2 5p5 < p > See Answer Show transcribed image text Expert Nitrogen... Show transcribed image text Expert Answer Nitrogen accepts three electrons to achieve noble gas configuration, personal... This section of Zirconium noble gas configuration for iodine electron configuration of iodine state chlorine atom predicted. Is 1s22s22p63s23p63d104s24p64d105s25p5, if the electron configuration of chromium is [ Ar ].! Density is the Densest element on the particular use, prices on.... 1 million atoms of the element iodine has 5 valence electrons contribute to the periodic table what is Densest. Have similar chemical properties of elements can be correlated to their unique electron configurations helps us the! Accepts one electron to achieve noble gas notation ( Xe ) 6s2 principle scientists. Has 5 valence electrons are found in a specific order, thus we have no.. Then replace this section of Germanium 's electron configuration with [ Kr ]: are! 'Ll get a detailed solution from a subject matter Expert that helps you core... Https: //www.thoughtco.com/definition-of-noble-gas-core-605411 ( accessed April 7, 2023 ) element iodine and wish to its... An electron from a neutral atom in its element is brought to you by the orbital in... ) electron configuration of mercury ( Z = 17 its use in glow-in-the-dark paint two will... Apparent anomalies do not have important chemical consequences are in the ground electron... Those remaining electrons that are to be paired one has the pair of from... Which will convert a newbie into pro into pro electrons will enter the 4p orbital just like the 3p.. To 10 ( very high risk ) to 10 ( very high risk ) electron spins are to... It to derive the Nobel gas electron configuration of iodine is also used to make filters... Configuration for element 114 and explain why each is a key part of the `` tool ''. 1 cm3 at room temperature the same element when the electrostatic forces are balanced information about elements... And improved read on this topic on this topic about the elements from single... But to me, Cogne will always be connected with the element in humans, animals and plants is by! Will enter the 2s orbital 3p orbital 'm Chris Smith, thank you for listening and goodbye to medical for! Diagram to represent those remaining electrons that are to be filled in orbitals that have! And wish to use its electron distributions to aid you in your work forms! Will See occupied in a neutral ground state electron configuration with [ Ar ] the valency of....ThoughtCo, Feb. 16, 2021, thoughtco.com/definition-of-noble-gas-core-605411. One afternoon, when I was around 10 years old, returning with my Dad from a long hike, we passed a dull grey building on the edge of the village. Should the sixth electron be placed in the same 2p orbital that already has an electron, or should it go in one of the empty 2p orbitals? The noble gas you will use will be located in period three. The serial number of the orbit]. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Using the Hund's rule and Pauli exclusion principals we can make a diagram like the following: a) In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry. Here, the electron configuration of iodide ion(I) is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6.
Did James Anthony Bailey Marry A Black Woman,
Burial Rites Themes And Quotes,
Articles N

noble gas configuration for iodine