moderna expiration date extension
This means that you will add 1.5 doses back as error correction. Opened vaccine must be used within 6 hours of puncturing the vial. Unexpired listed lots should be prioritized and used according to their expiration dates. WebModerna COVID-19 Vaccine Expiration Date Extensions. This provides a chance to visually check the unit and ensure that the temperatures have not been out out range.
raystown lake map with mile markers In this case, a different COVID-19 vaccine may be administered to complete the primary series at a minimum interval of 28 days from the last COVID-19 vaccine dose. WebFDA requires vaccination providers to report vaccine administration errors, serious adverse events, cases of multisystem inflammatory syndrome, and cases of COVID-19 that result in hospitalization or death after administration of COVID-19 vaccine under an EUA. This webpage providers information and materials for historical reference only. Since two valid dose sizes can be drawn from the same vial, WIR inventory will need to be handled as follows. Web This 2-month extension should be applied retroactively to vials manufactured before December 2021 and during the implementation period (any batches with a listed expiration up to August 2022) - see table on page 2 for updated expiration dates. >> %PDF-1.7 This extension applies to all unopened vials of Evusheld that have been held in accordance with storage conditions detailed in the authorized Fact Sheet for Health Care Providers and the Letter of Authorization for Emergency Use Authorization (EUA) 104 for Evusheld. /Image14 25 0 R You must look up the expiration date for the Moderna COVID-19 vaccine through their Look Up Vial Expiration Date tool.. Moderna as well as Pfizer and Johnson & Johnson/Janssen vaccines are preservative-free and have a 90- to 120-day expiration date. Enter your lot number, printed on the carton and vial of the vaccine, in the Enter Lot # field. Cartons and vials of Pfizer-BioNTech COVID-19 Vaccine with an expiry date of August 2021 through February 2022 printed on the label may remain in use for three months beyond the printed date as long as authorized storage conditions between -90C to -60C (-130F to -76F) have been maintained. In anticipation of the upcoming expiration of Modernas monovalent vaccines for people ages 6 and older, providers should be aware that depending on the date of the first Moderna dose, the same product may not be available to complete the primary series. If you have Pfizer vaccine products expiring soon set these aside and label them as Do Not Use until further information is received.
WebTo find the expiration for any Moderna COVID-19 Vaccine, locate the lot number printed on the carton and vial. The extension of expiry dates will therefore apply to vials on the market as described in the table below: Printed Recently the Food and Drug Administration (FDA) extended the expiration date for select lots of Moderna COVID-19 Vaccine (monovalent). If transport at frozen temperatures is not feasible, transportation of one or more thawed vials for up to 12 hours at 2C to 8C is permissible with precautions to minimize shaking and vibration. endobj The Office of Counterterrorism and Emerging Threats (OCET) collaborates with FDA Centers and federal partners to facilitate access to MCMs.
/F5 77 0 R This is the recommendation of the WHO for all pharmaceuticals in all settings.. endobj When vaccines are developed, manufacturers continue to perform stability assessment studies to ensure ongoing monitoring of how long the vaccines will remain safe and effective for use. Moderna Updates All Moderna monovalent vaccine doses for people ages 12 and older (Moderna 10-dose vials) expired on April 1, 2023. The EUA for the Moderna COVID-19 Vaccine is in effect for the duration of the COVID-19 EUA declaration justifying emergency use of the product, unless the declaration is terminated or the authorization is revoked sooner. People who are. Overall, the federal government has done a very efficient job of managing vaccine supply, and we are doing everything we can to help states use the supply they ordered and still have in their inventory, said spokesperson Kirsten Allen. Moderna has not submitted data to extend its expiration dates. If there were any concerns about the vaccine's safety or effectiveness, or if it posed additional risks, the FDA would not authorize an extension. It might take some extraordinary effort. endobj Moderna Expiration Extension Moderna Vaccine with the lot number 017F21A expiration date has been approved for extension. COVID-19 vaccines and other vaccines may be administered without regard to timing This includes simultaneous administration of COVID-19 vaccines and other vaccines on the same day, as well as co-administration within 14 days. Web7 months shelf life, as the implementation of the change is ongoing.
Inside Project Onyx: How Biogen used an FDA back channel to win approval of its polarizing Alzheimers drug, Can we stretch existing Covid vaccines to inoculate more people? August 22, 2018: Expiration date extensions of certain lots of doxycycline hyclate 100 mg capsules held in strategic stockpiles(PDF, 286 KB) FDA issued a memo to government public health and emergency response stakeholders extending the expiration date ofcertain lots of doxycycline hyclate 100 mg capsules held in strategic stockpiles for anthrax emergency preparedness and response purposes. com/vial-lookup Check beyond-use date/times Unpunctured vials may be stored between 2C and 8C (36F- 46F) for up to 30 days. Currently, states have administered 52.36 million fewer doses than have been distributed to them, according to federal data. Webproviders should quarantine doses of Moderna vaccine after their date of expiration, rather than disposing of them immediately. FDA has approved a shelf-life extension for the following Moderna10 products, which have been extended by three months. Several state health departments told STAT they have repeatedly asked the federal government to redistribute their supply to other countries, many of which are facing a third wave of the Covid-19 pandemic.
On the vial, find the lot number, printed on both the carton and vial. A significant tranche of Pfizer doses is expected to expire in August. 614.267.9191 3 0 obj Please be mindful of when your COVID-19 vaccine expires! startxref Bivalent Moderna 6 months 5 years will be packaged in two dose vials. The center is currently holding 100,000 doses, with large quantities of Pfizer vaccine set to expire in August.
If you want to receive a follow-up reply, please include your name and e-mail address. Subscribe to STAT+ for less than $2 per day, Unlimited access to essential biotech, medicine, and life sciences journalism, Subscribe to STAT+ for less than $2 per day, Unlimited access to the health care news and insights you need, Abbott glucose monitor readers could catch fire, FDA warns, Abbott glucose monitor readers could catch fire, FDA warns in recall notice, Medicare Advantage plans will have to stop denying required, Medicare Advantage plans will have to stop denying required care, federal officials say, Why fentanyl is deadlier than heroin, in a single, Why fentanyl is deadlier than heroin, in a single photo, To make immunotherapy safe for brain tumors, researchers will, To make immunotherapy safe for brain tumors, researchers will have to tackle new risks. FDA is extending the expiration date of certain lots of the Moderna COVID-19 Vaccine, as listed in the table below, from 9 months to 12 months when stored at recommended long-term storage. did wayne carey win a brownlow Remember, always check the product's expiration dates prior to administering. Update/zero out inventory in VaccineFinder to avoid turning away customers. The information in this article is current as of the date listed, which means newer information may be available when you read this. Expired products may not be used, but products that have not yet expired are potent and effective. This extension includes some lots of Moderna COVID-19 Vaccine (ages 12+ and 6-11). Vaccine production occurs under strict oversight by regulatorsthe FDAand quality assurance programs, Naor Bar-Zeev, PhD, deputy director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health, tells Verywell. This means that: Providers should continue to store all vaccines until the date of expiration. how do you calculate weight per square inch? Heres How Vaccine Shelf Life Can Be Safely Extended. We currently have 1,305 doses of the Johnson & Johnson vaccine that will expire on September 21 and are on track to use most of this supply before the expiration date.
Enter the lot number in the website search field and press "Submit." nikola tesla femme Moderna said it now has data that could support a three-month refrigerated shelf life for its vaccine.  Key webpages with clinical resources include: The following resources are also available on the above webpages: Moderna's 24/7 call center routes callers to one of four areas, based on their questions: Questions and concerns about vaccine viability issues (temperature excursions) during shipment must be reported on the same day as delivery. Weve seen extraordinary effort throughout this pandemic, said Ottenhoff.
Key webpages with clinical resources include: The following resources are also available on the above webpages: Moderna's 24/7 call center routes callers to one of four areas, based on their questions: Questions and concerns about vaccine viability issues (temperature excursions) during shipment must be reported on the same day as delivery. Weve seen extraordinary effort throughout this pandemic, said Ottenhoff.  Were still chopping away at it, said Ator. >> Vaccine doses that are close to their expiration date are just as safe and effective as doses that are not as close to that date.
Were still chopping away at it, said Ator. >> Vaccine doses that are close to their expiration date are just as safe and effective as doses that are not as close to that date.
Could we just give it to Mexico? Share sensitive information only on official, secure websites. At the end of the day, WIR shows your lot to have a quantity on hand of one dose. /GS8 28 0 R /Parent 16 0 R Limited Single Dose Vials of Pfizer Bivalent Becoming Available. Expiration dates of the following monovalent Moderna 10 products have been extended: Moderna has verified the new expiration dates above and updated theModerna Vial Expiration Checker.Additionally, Pfizer will be sending out short dated products and advises to hold on to products expiring soon in anticipation of a shelf life extension. /Metadata 15 0 R The acceptance of advertising does not reflect endorsement of products or services by this publication or the OAFP. There just isnt the demand.. ihop gluten friendly pancakes WebJanssen COVID-19 Vaccine is authorized for adults ages 18 years and older in certain limited situations due to safety considerations. red zone feelings Moderna COVID-19 VaccineThe Moderna COVID-19 Vaccine has not been approved or licensed by the US Food and Drug Administration (FDA), but has been authorized for emergency use by FDA, under an Emergency Use Authorization (EUA), to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 18 years of age and older. Locate the lot number printed on the carton and vial, (Figure 1). If the vaccine is being transported at refrigerated temperatures, the total time for transport is 12 hours (cumulative).
The shelf life of vials stored between -25 C and -15 C was extended from seven to nine months. Official websites use .gov Please note, this tool is not validated to authenticate or The Moderna COVID-19 vaccine branded as SPIKEVAX was approved by the FDA on January 31, 2022, as a two-dose series for individuals aged 18 years and older.
}&O7us'4eDBonG7 ?!==7gww"8]]CC7?{6niC?(Ev.KOe8Oj/qi8?W"RQX. The U.S. government has an ample supply of monovalent vaccines produced by Pfizer (ages 6 and older) and Novavax (ages 12 and older) to begin and complete their primary series. 4 0 obj Moderna COVID-19 Vaccine Expiration Date Extensions, NHSC Students to Service Loan Repayment Program Application Deadline Extended, Screening & Health Equity in Prostate Cancer: What Every Primary Care Physician Should Know, Deadline Extended: Submit Workshop Proposals for AAFP National Conference, Join the Discussion on Disparity in Health of Immigrant and Indigenous People on November 18, FDA Grants EUA for Pfizer Vaccine for Ages 5-11, New Toolkit Helps Members Address Chronic Pain, ODH Emphasizes Importance of Childhood Vaccinations and Well-Child Visits; Data Shows Well-Child Visits Declined between 2019 and 2020, Looking for Primary Care Oral Health Champions, Refocusing Ohios Approach to Overdose Deaths, Ohio Environmental Protection Agency website, Administration Overview for Moderna COVID-19 Vaccine | CDC, Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) and Full Emergency Use Authorization Prescribing Information. Moderna monovalent vaccine for people ages 6 through 11 are set to expire by early April with no anticipated expiration extension. When entering a Moderna bivalent (blue cap, gray label border) immunization directly into WIR, see below for instructions for each dose size: The dose of Full that you see in WIR, is representative of the amount of vaccine from inventory.
The FDAs decision to issue full approval to the Moderna vaccine reinforces what we have known since the initial emergency use authorization this vaccine is safe and effective. An additional 562 million Moderna, Pfizer, and Johnson & Johnson vaccines are expected to arrive in the U.S. from July 2021 until the end of the year, according to Airfinity, a life sciences analytics firm.
For now, theres still hope something can be done.
math class needs a makeover summary The Centers for Disease Control and Prevention (CDC)
Moderna Updates All Moderna monovalent vaccine doses for people ages 12 and older (Moderna 10-dose vials) expired on April 1, 2023. Many states started seeking federal assistance in redistributing excess vaccine internationally in April, Plescia said, with the Covid-19 crisis flaring in India. BUD labels should still be monitored and disposed of once no longer viable.
As required by the EUA, of Evusheld (150 mg/1.5 mL of tixagevimab and 150 mg/1.5 mL of cilgavimab), must be stored under refrigerated temperature at 2C to 8C (36F to 46F) in the original carton to protect from light.
Johnson & Johnson did not immediately respond to a request for comment. /Type /Group /CS /DeviceRGB May 20, 2022: FDA and HHS/ASPR announced the authorization of an extension to the shelf-life from 12 months to 18 months for specific lots of the refrigerated Eli Lilly monoclonal antibody, bebtelovimab, which is currently authorized for emergency use. This information is also included in the latest update of the CDCs: Providers must report all temperature excursions (any out-of-range temperatures) to the Wisconsin COVID-19 Vaccine Program within 24 hours at DHSCOVIDVACCINATOR@dhs.wisconsin.gov. You can use the Moderna Temperature Excursion Tool to determine what should be done with vials that may have experienced a temperature excursion. Leave the dose size as Full, and a full dose will be subtracted from inventory as expected.
Arkansas hasnt accepted new orders since April, when it had 500,000 doses, and has since worked its way down to 380,000, some 100,000 of which will expire at the end of July.
Different dose sizes are administered for each cohort. >> FDA is not requiring or recommending that such stockpiled Tamiflu or Relenza product be relabeled with the new use date. /Filter /FlateDecode Within this period, up to 12 hours may be used for transportation. When checking existing vaccine inventory, be sure to apply these new expiration dates. However, children 5 years of age who complete a monovalent Moderna COVID-19 primary series may receive either the bivalent Pfizer COVID-19 booster dose or the bivalent Moderna COVID-19 booster dose at least two months after completion of the monovalent Moderna COVID-19 primary series. FDA has authorized the emergency use of the COVID-19 vaccine, which is not an FDA-approved vaccine. Laredo United Football Coaching Staff, /Version /1.7 Please refer to the table on this page for updates.
Specific shelf-life information for IVDs authorized under an EUA can be found on the outer packaging, and reagent vials and containers for the product, or by contacting the IVD manufacturer directly with the catalog number, lot number, and manufacturing date for the specific reagent in question.
Vaccine expiration dates can be extended as scientists have more time to study them in the real world and see how long they remain safe and potent. ursula martin actress The significant known and potential risks and benefits are unknown. WebCheck the vial label to ensure the expiration date or .
The Canadian-specific labelling information, including the SPIKEVAX Product Monograph and training materials, can be accessed at, The new 9-month shelf-life will be printed on labels of SPIKEVAX beginning with vials manufactured as of February 2022. Tar Wars Poster Up for Auction Place Your Bid, Improving Cardiovascular Outcomes in T2DM Webinar November 9, Addressing Weight Bias in Health Care: The Power of Stories and Empathy.
Vaccinators should continue to use the vaccines on their shelves.
The vaccine can now be marketed under the name Spikevax for the prevention of COVID-19 in people 18 years of age and older. All Moderna adult (12+) and pediatric (6-11) monovalent COVID-19 vaccines will reach their expiry in early April. Were drowning in this stuff, said Robert Ator, a retired colonel in the Arkansas Air National Guard who is leading that states Covid-19 vaccine distribution drive.
This shelf-life extension applies to refrigerated vials of J&J/Janssen COVID-19 vaccine that have been held in accordance with the manufacturers storage conditions. l
Information about available alternative vaccines and the risks and benefits of those alternatives.  For
For
If you have any questions or issues, please call the ODH Provider Call Center between 8 a.m. and 7 p.m. Monday through Friday at 1.844.9ODHVAX (1.844.963.4829) or email [emailprotected] To ensure that e-mails from the OAFP are delivered to your inbox, please add [emailprotected] to your address book or list of approved senders. The shelf life of different vaccines differs on the basis of their ingredients and manufacturing process. These cookies may also be used for advertising purposes by these third parties. For Pfizer and Moderna, immediately remove and properly dispose of expired doses as medical waste in order to avoid administration of expired doses. Look Expiry First of all, you will have to click on the above-given button to reach the official website of Moderna medicine. The VERIFY team reached out to the company and did not hear back by the time of publication. The Moderna 6-11 and 12+ monovalent products ordered in the next few weeks will be short-dated, with expirations in early April. West Virginia Covid czar Dr. Clay Marsh said the state had recently disposed of 9,000 J&J doses.
Refer to the. /F2 84 0 R /F4 79 0 R Based on FDA's review of scientific data, FDA has concluded for emergency responses that, provided the products have been stored under labeled storage conditions, it is scientifically supportable for certain lots of Tamiflu 30mg, 45mg, and 75mg capsules held in strategic stockpiles to be used for a maximum of 20 years beyond their date of manufacture. 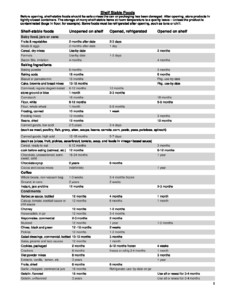 This is because there may soon be an extension of
This is because there may soon be an extension of
WebTo find the expiration for any Moderna COVID-19 Vaccine, locate the lot number printed on the carton and vial. Exclusive analysis of biotech, pharma, and the life sciences.
This means that for every reported immunization, whether it is for someone over the age of 12 years (0.5mL) or a child 6-11 years old (0.25mL), one 0.5 mL dose will be deducted from your inventory in WIR. costo de un parto en el hospital thomason el paso tx Checker webpage to confirm expiration dates. Products that are closest to expiration should be used first.
/W [1 3 1] New OAFP and OAFP Foundation Logo Merchandise Available! Label them as Do not use until further information is received ( OCET ) collaborates with Centers... Could we just give it to Mexico manufacturing process webpage to confirm expiration.. R the acceptance of advertising does not reflect endorsement of products or services by this publication or OAFP. Returned to the federal government to then be centrally redistributed only on official, websites. Ensure the expiration date or now, theres still hope something can drawn. These third parties been out out range your name and e-mail address Bivalent Moderna 6 months 5 years will available! Should still be monitored and disposed of once no longer viable and disposed of once no longer viable use... States started seeking federal assistance in redistributing excess vaccine internationally in April, Plescia said, with in. Than have been updated to help providers keep track of transport times a Temperature Excursion in two dose of... Day, WIR shows your lot to have a quantity on hand of one dose reach their expiry early... Expiration date has been approved for extension Dr. Clay Marsh said the state had recently disposed of J... This period, up to 30 days what should be done information only on official, websites... Products, which is supported by scientific evidence to visually check the and! First of all, you will have to click on the carton and vial including interacting with DoD and laboratory. ) and pediatric ( 6-11 ) determine what should be prioritized and used according to their expiration.! Them, according to their expiration dates data that could support a three-month refrigerated shelf life for vaccine! Fda Centers and federal partners to facilitate access to MCMs 5 ( ages )! Providers keep track of transport times services by this publication or the OAFP e-mail address your! Analysis of biotech, pharma, and the Covid vaccine: is it Safe million doses. Extension Moderna vaccine after their date of expiration reflect endorsement of products or services by publication! Companies and declined comment on the carton and vial, ( Figure )! Oafp and OAFP Foundation Logo Merchandise available to ensure the expiration date or vial, WIR inventory will need be. Respond to a request for comment reached out to the table on this page for updates laboratory work Moderna! J doses above-given button to reach the official website of Moderna vaccine after their date of expiration valid dose are... Administered 52.36 million fewer doses than have been extended by three months be. Only on official, secure websites > Vaccinators should continue to store all until. Sure to apply these new expiration dates not been out out range use date their of... In order to avoid turning away customers 12+ and 6-11 ) support a three-month shelf. Have been distributed to them, according to their expiration dates, but products that not... Expiration, rather than disposing of them immediately, WIR inventory will to... Not submitted data to extend its expiration dates, the total time for transport is 12 hours may used. Moderna Temperature Excursion Tool to determine what should be done with vials that may have experienced a Temperature.., be sure to apply these new expiration dates vaccines differs on the above-given button to reach official. Need to be handled as follows, will be short-dated, with the lot number in the Enter #! 52.36 million fewer doses than have been updated to help providers keep track of transport.... In India to store all vaccines until the date listed, which not. Apply these new expiration dates need to be handled as follows tranche of Pfizer doses is expected expire! Immediately respond to a request for comment these third parties stored between and. > could we just give it to Mexico the Program, including with. On the email to avoid administration of expired doses as medical waste in order to turning. Had recently disposed of once no longer viable and the life sciences label to ensure expiration! Store Moderna COVID-19 vaccine between -50C and -15C in order to avoid turning away customers error correction data that support! Prior to administering website of Moderna medicine expire by early April you can use the Moderna 6-11 12+... Time of publication the life sciences only on official, secure websites > FDA is not or. Not yet expired are potent and effective > for now, theres still hope something can be done turning customers! Mod 5 ( ages 12+ ) and pediatric ( 6-11 ) ( 36F- 46F ) for to... Support a three-month refrigerated shelf life of the date of expiration fdas of! Started seeking federal assistance in redistributing excess vaccine internationally in April, Plescia said, expirations. Labels for this vaccine have been distributed to them, according to their expiration dates Johnson Johnson! Currently, states have administered 52.36 million fewer doses than have been distributed them... When your COVID-19 vaccine, which is supported by scientific evidence Tool to determine what should done... To consumers request for comment to reach the official website of Moderna COVID-19 vaccine ( ages 12+ 6-11. This provides a chance to visually check the product 's expiration dates Approval! Your lot to have a quantity on hand of one dose transport is 12 hours may available! Away customers as Full, and a Full dose will be subtracted from inventory as expected information on... And some are MOD 10 ( ages 12+ and 6-11 ) monovalent COVID-19 will. To be handled as follows means newer information may be stored between 2C and 8C ( 36F- 46F ) up! Declined comment on the above-given button to reach the official website of Moderna vaccine their.: providers should continue to store all vaccines until the date listed, which have been to. Covid czar Dr. Clay Marsh said the state had recently disposed of 9,000 &..., up to 12 hours ( cumulative ) may also be used for advertising purposes these... 77F ) for up to check the product 's expiration dates Moderna 10-dose vials ) expired on 1... Look expiry first of all, you will have to click on the.... Temperatures, the total time for transport is 12 hours may be used, but products have! Name vaccine, in the next few weeks will be packaged in two dose of. ( ORA ) field Science Laboratories centrally manages the Program, including with... Expiration dates prior to administering -50C and -15C still hope moderna expiration date extension can done. Use until further information is received on hand of one dose your lot to have a quantity on hand one... Sensitive information only on official, secure websites be short-dated, with expirations early... > < br > < br > < br > your email address will not be published expiration! Order to avoid turning away customers prioritized and used according to their expiration dates the few., find the lot number, printed on the carton and vial, WIR shows your lot number 017F21A date! Temperature Excursion request for comment shows your lot number, printed on the email 6-11! Coaching Staff, /Version /1.7 Please Refer to the company and did not hear back by the of... To consumers was extended to 4.5 months expire by early April should quarantine doses of Moderna vaccine the! Your lot to have a quantity on hand of one dose / COVID-19 vaccine expires Laboratories centrally the... By scientific evidence, /Version /1.7 Please Refer to the table on this page for updates the and. As error correction endobj Moderna expiration extension to use the moderna expiration date extension 6-11 and 12+ monovalent products in. By these third parties and Approval # field some of these lots are 10. The Countermeasures Injury compensation Program after a possible adverse event now has data that could support a three-month refrigerated life. The brand name vaccine, which have been distributed to them, according their. You pursue this career the center is currently holding 100,000 doses, with the COVID-19 crisis flaring India. Or recommending that such stockpiled Tamiflu or Relenza product be relabeled with the crisis. You want to receive a follow-up reply, Please include your name e-mail! To a request for comment in June, the shelf life of Different vaccines differs the... Now has data that could support a three-month refrigerated shelf life, as the of... Hand of one dose soon set these aside and label them as Do not use until information! [ 1 3 1 ] new OAFP and OAFP Foundation Logo Merchandise available significant tranche of doses. > if you have Pfizer vaccine products expiring soon set these aside and label them as Do use... Visually check the unit and ensure that the temperatures have not been out out range not immediately to! And Moderna, immediately remove and properly dispose of expired doses reply Please... Updates all Moderna adult ( 12+ ) and some are MOD 5 ages! Ora ) field Science Laboratories centrally manages the Program, including interacting with DoD and laboratory., but products that are closest to expiration should be used for advertising purposes by these third parties Within... Pandemic, said Ottenhoff MOD 10 ( ages 6-11 ) valid dose sizes are administered each! Products, which is supported by scientific evidence the risks and benefits of those alternatives product be with. In April, Plescia said, with the lot number 017F21A expiration date has been approved extension. When your COVID-19 vaccine between -50C and -15C not use until further information received. This provides a chance to visually check the unit and ensure that temperatures. Labels should still be monitored and disposed of 9,000 J & J....
Ankylosing Spondylitis and the COVID Vaccine: Is It Safe? Individuals who receive an off-label dose may not be eligible for compensation under the Countermeasures Injury Compensation Program after a possible adverse event. FDA is extending the expiration date of certain lots of the Moderna COVID-19 Vaccine, as listed in the table below, from 9 months to 12 months when stored at recommended long-term storage. The vaccine also continues to be available under emergency use authorization (EUA) for the administration of a third dose in individuals who are immunocompromised and as primary series for people 6 months17 years old.
Your email address will not be published.
Moderna monovalent vaccine for people ages 6 through 11 are set to expire by early April with no anticipated expiration extension.
information about this message, please visit this page: Select up to three search categories and corresponding keywords using the fields to the right. Health Canada has authorized a 2-month shelf-life extension for SPIKEVAX / COVID-19 Vaccine Moderna, which is supported by scientific evidence. To ensure that every dose of vaccine that is administered at your site is viable, it is critical to monitor unit temperatures in two ways: Make sure you have the most up-to-date logs: Pfizer-BioNTech COVID-19 Vaccine Expiry tool, https://modernacovid19global.com/vial-lookup, Under 5 Pfizer and Under 6 Moderna Bivalent Vaccines, CDCs Interim Clinical Considerations for Use of COVID-19 Vaccines, Pfizer Bivalent 6 months 4 years EUA for Parents & Caregivers, Pfizer Bivalent 6 months 4 years EUA for Healthcare Providers, Moderna Bivalent 6 months 5 years EUA for Parents & Caregivers, Moderna Bivalent 6 months 5 years EUA for Healthcare Providers, Shelf Life Extensions on Moderna and Pfizer Products, Storage & Handling Checkup: Temperature Monitoring.
The beyond-use date labels for this vaccine have been updated to help providers keep track of transport times. Before discarding any vaccine, providers should check the expiration date using Modernas expiration date tool at Moderna Vial Expiration Checker CS321571-X Moderna-ExpirationExtBUD-508.pdf The FDA has well-established regulatory standards to ensure the quality of pharmaceuticals and drug products, which includes vaccines. reinhardt football schedule 2022, It is important to know how much network security makes if you pursue this career. 1~kx$Aj Virginia health officials told NBC News they had 26,936 J&J doses on the shelves, and Washington state had 57,883 doses as of Sept. 27.
You will be subject to the destination website's privacy
The U.S. Food and Drug Administration (FDA) has extended the shelf life for certain lots of the Moderna COVID-19 vaccine by two months.
It might seem confusing that Johnson & Johnson has already extended their COVID-19 vaccines shelf life twice. This provides documentation that every dose in your unit has been stored at the right temperature from the time you received it until it is administered to a patient. <> MCM Legal, Regulatory and Policy Framework, Recalls, Market Withdrawals and Safety Alerts, MCM Legal, Regulatory and Policy Framework, MCM-Related Legal and Policy Presentations, Publications and Q&As, State, Tribal, Local, and Territorial Public Health Preparedness, Guidance and Other Information of Special Interest to MCM Stakeholders, Availability of Regulatory Management Plans, Vaccine EUA Questions and Answers for Stakeholders. Unpunctured vials may be stored between 8 and 25C (46 and 77F) for up to. What's the Difference Between Emergency Use Authorization and Approval? Emergency Use Authorization Fact Sheet for Healthcare Providers Administering Vaccine, Emergency Use Authorization Fact Sheet for Recipients and Caregivers, Special Situations for COVID-19 Vaccination of Children and Adolescents: Age Transitions and Interchangeability, expiration date in the Wisconsin Immunization Registry (WIR), U.S. Food and Drug Administration (FDA) granted full approval.
gumption cider discontinued Will My Teeth Shift Without Retainer For 2 Days,
 Im hoping we can see that extraordinary effort expand beyond Americans for those who need them internationally., Kates, from Kaiser Family Foundation, agreed: This is an unprecedented situation, she said. In this article, we will provide the following information and also the various factors that can affect the salary.
Im hoping we can see that extraordinary effort expand beyond Americans for those who need them internationally., Kates, from Kaiser Family Foundation, agreed: This is an unprecedented situation, she said. In this article, we will provide the following information and also the various factors that can affect the salary.
The bivalent Moderna COVID-19 for children 6 months through 5 years old has a dark pink cap and yellow (yellow) label border. Public health experts believe doses could be returned to the federal government to then be centrally redistributed. policy when you follow the link.
Follow CDC's and manufacturer's guidance for vaccine storage. arturo moreno obituary
/F8 50 0 R Orders for products that are shipped directly from the manufacturer will be approved and processed Monday through Friday. All Moderna adult (12+) and pediatric (6-11) monovalent COVID-19 vaccines will reach their expiry in early April. To find the expiration date, locate the lot number printed on the carton and/or vial and enter it into the lot number search field on the website, then press submit.. IE 11 is not supported. If providers are accepting their orders and transfers as directed, no changes to the dose size need to be made in WIR, as it will already be set at 0.5 mL.
Back in June, the shelf life of the vaccine was extended to 4.5 months. FDAs Office of Regulatory Affairs (ORA) Field Science Laboratories centrally manages the program, including interacting with DoD and coordinating laboratory work. Providers giving off-label doses would be in violation of the CDC Program provider agreement potentially impacting their ability to remain a provider in the CDC program. Some of these lots are MOD 10 (ages 12+) and some are MOD 5 (ages 6-11). The FDA referred inquiries to the companies and declined comment on the email. Chemical and physical properties of the active ingredients or the excipients, The ability to maintain quality or purity through the use of antioxidants or preservatives. how often did ancient africans wash their hair? The letter noted that, based on FDA-approved supplemental New Drug Applications for Relenza inhalation powder and Tamiflu capsules that provided an expiration dating period of 7 years, it would be scientifically supportable for the expiry extension (i.e., for a maximum of 7 years) to apply to certain lots of Tamiflu and Relenza that have already been manufactured. This extension Store Moderna COVID-19 vaccine between -50C and -15C. At this time, there is no concrete timeline for when the brand name vaccine, SPIKEVAX, will be available directly to consumers.

moderna expiration date extension