standard enthalpy of formation of hexane
National Institute of Standards and Thermodynam., 1988, 20, 859-865. VI. Heats of combustion and formation of the paraffin hydrocarbons at 25 C, What is the standard enthalpy of formation (AHp) of liquid CoH14 given that the standard enthalpy of formation of CO2(g) is -394 kJ/mole and H2O(l) is -286 kJ/mole? Chem., 1986, 64, 2139-2141. Data Program, but require an annual fee to access. = 36.29 kJ(found here). Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. Soc., 1936, 58, 146-153. [Total 3 marks] 7. ; Marsicano, F., 1. ; Lacey, W.N., The standard heat of formation of any element in its most stable form is defined to be zero.
Chem., 1951, 43, 946-950. [all data], Huffman, Parks, et al., 1931 [all data], Rogers and Siddiqui, 1975 The Heats of Vaporization of Some Hexanes 1, ; Benson, G.C., 0.444 J/gC Willingham, C.B. Vyssh. Data compilation copyright We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. By formula: C5O5W(g)+C6H14(g) = C11H14O5W(g), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Gas phase ion energetics data, References, Notes, kH(T) = kH exp(d(ln(kH))/d(1/T) ((1/T) - 1/(298.15 K))) Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, WebEnthalpy of combustion of liquid at standard conditions: f H gas: Enthalpy of formation of gas at standard conditions: f H liquid: Enthalpy of formation of liquid at standard [all data], Czarnota, 1979
Given a simple chemical equation with the variables A, B and C representing different compounds: and the standard enthalpy of formation values: the equation for the standard enthalpy change of formation is as follows: Hreactiono = Hfo[C] - (Hfo[A] + Hfo[B]), Hreactiono = (1 mol)(523 kJ/mol) - ((1 mol)(433 kJ/mol) + (1 mol)(-256 kJ/mol)\). in these sites and their terms of usage. [all data], von Reis, 1881 ; Badalov, Yu.A., Enthalpy of vaporization (at saturation pressure) Soc., 1973, 95, 8605-8610. Be prepared. Acad. Unsmoothed experimental datum given as 2.356 kJ/kg*K.; T = 293 to 324 K. Unsmoothed experimental datum given as 2.276 kJ/kg*K.; T = 185 to 300 K. Unsmoothed experimental datum. (TRC) data available from this site, much more physical To find the Hreactiono, use the formula for the standard enthalpy change of formation: The relevant standard enthalpy of formation values from Table 1 are: Plugging these values into the formula above gives the following: \[H_{reaction}^o= (2 \cancel{mol})(33.18\; kJ/\cancel{mol}) - \left[(2 \cancel{mol})(90.25\ kJ/\cancel{mol}) + (1 \cancel{mol})(0\; kJ/\cancel{mol})\right]\]. Good, W.D. Soc., J. Chem. Zaved., ; Roux-Desgranges, G.; Grolier, J.-P.E., Therefore, \(\ce{O2(g)}\), \(\ce{H2(g)}\), and graphite have \(H^o_f\) values of zero.
Using the values in the above table of standard enthalpies of formation, calculate the Hreactiono for the formation of NO2(g). Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. The standard enthalpy of complete combustion of liquid hexane (C6H14) is -4163 kJ/mol. The figure shows two pathways from reactants (middle left) to products (bottom). Specific Enthalpy= Specific Entropy = Molar Volume Fraction= Saturated reply. ; D'Arcy, P.J., The enthalpy change of combustion of hexane was measured using a calorimeter containing 200 cm3 of water; 0.5g of hexane (C6H14) was burnt. [all data], Potzinger and Bunau, 1969 Heat capacities of liquids at temperatures between 90 and 300 K and at atmospheric pressure. ; Yerlett, T.K., Example #1: Calculate the standard enthalpy of combustion for the following reaction: Before launching into the solution, notice I used "standard enthalpy of combustion." Williamham, C.B. ; Snelson, A., Am. The symbol of the standard enthalpy of formation is Hf. [all data], Parks, Huffman, et al., 1930 Multiplying both \(\ce{H2(g)}\) and \(\ce{Cl2(g)}\) by 1/2 balances the equation: \[ \ce{1/2 H_{2} (g) + 1/2 Cl_{2} (g) \rightarrow HCl (g)} \nonumber\], The standard states of the elements in this compound are \(\ce{Mg(s)}\), \(\ce{C(s, graphite)}\), and \(\ce{O2(g)}\). WebThe standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure elements. J. Quant. C (s, graphite) + O2 (g) CO2 (g) Calculate the specific heat capacity for a 13.7-g sample of nickel that absorbs 361 J when its temperature increases from 28.4 C to 87.8 C. by the U.S. Secretary of Commerce on behalf of the U.S.A. J. Phys. Mass Spectrom.
[all data], Scott D.W., 1974, 2 Grolier, J.P.E. The standard enthalpy of reaction (\(H^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of formation of the reactants (each multiplied by its stoichiometric coefficient)the products minus reactants rule. ; Huffman, H.M.; Thomas, S.B., ; D'Arcy, P.J. Douslin, D.R. Phys., 1961, 34, 189.
Ohnishi, K.; Fujihara, I.; Murakami, S., The standard enthalpy of reaction \(\Delta{H_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. Stull, D.R.,  Am. The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Zaripov, Z.I., Prosen, E.J. [all data], Connolly, Sage, et al., 1951
Am. The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Zaripov, Z.I., Prosen, E.J. [all data], Connolly, Sage, et al., 1951
Fractional coefficients are OK. Radiative Transfer, 1962, 2, 369. ; Uncertainty assigned by TRC = 0.007 l/mol; Based on data from 286. log10(P) = A (B / (T + C)) ; Rastorguev, Yu.L.
[ 4 ], and was also used for the initial development of high-accuracy ANLn composite electronic structure methods [ 5 ]. The symbol of the standard enthalpy of formation is H f. = A change in enthalpy; o = A degree signifies that it's a standard enthalpy change. Chem. Michou-Saucet, Marie-Annie; Jose, Jacques; Michou-Saucet, Christian; Merlin, J.C., { "7.1:_Nature_of_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
I. Kalinowska, B.; Jedlinska, J.; Woycicki, W.; Stecki, J., Heat capacities of binary mixtures of n-heptane with hexane isomers, Correlation of the chemical thermodynamic properties of alkane hydrocarbons, J. Chem. 
the By the way, this is a common test question. All rights reserved. [all data], Grigor'ev and Andolenko, 1984 ; Smith, N.K., [all data], Skinner and Snelson, 1959
n-Hexane, methylcyclopentane, and n-octane, ; Benson, G.C., DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Condensed phase thermochemistry data, Notes, Good and Smith, 1969 J. Chem. . [all data], Waddington and Douslin, 1947 [all data], Waddington G., 1947 DRB - Donald R. Burgess, Jr. Using the axes below, show the enthalpy profile diagram for the formation of hexane. These are the conditions under which values of standard enthalpies of formation are typically given. Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. WebThe enthalpy change when 1 mol of hexane is formed from its constituent elements in their standard states under standard conditions. Eng. Faraday Trans., 1986, 1 82, 2977-2987. WebThe enthalpy change for this reaction is 5960 kJ, and the thermochemical equation is: C12H22O11 + 8KClO3 12CO2 + 11H2O + 8KCl H = 5960kJ Check Your Learning When 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of FeCl 2 ( s) and 8.60 kJ of heat is produced.
Eng.
Thermodynam., 1988, 20, 859-865. To determine which form is zero, the more stable form of carbon is chosen. The general equation for the standard enthalpy change of formation is given below: Plugging in the equation for the formation of CO2 gives the following: Hreactiono= Hfo[CO2(g)] - (Hfo[O2(g)] + Hfo[C(graphite)]. Thermodynamics of (1-chloronaphthalene + n-alkane): excess enthalpies, excess volumes and excess heat capacities, Make sure you find it and figure out how to use it. We can also measure the enthalpy change for another reaction, such as a combustion reaction, and then use it to calculate a compounds \(H^_f\) which we cannot obtain otherwise. [all data], Costas and Patterson, 1985 What is the standard enthalpy of formation (AHp) of liquid CoH14 given that the standard enthalpy of formation of CO2 (g) is -394 kJ/mole and H2O (l) is -286 kJ/mole? The standard enthalpies of formation are: NO(g) = +90.3 kJ/mol and HO(g) = -241.8 kJ/mol. [all data], Grolier, Inglese, et al., 1981 ; Yanin, G.S.,
J. , and was also Die Berechnung von Resonanzenergien; das MM2ERW-Kraftfeld,
Am. [all data], Scott D.W., 1974 ; Liu, R., Data, 1963, 8, 3, 371-381, https://doi.org/10.1021/je60018a027
Fluid Phase Equilib., 1989, 46, 59-72. Both the liquid an the vapor are flammable. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Wormald, C.J. [all data], Boublik, Fried, et al., 1984 ; Ruhoff, J.R.; Smith, H.A. Heat capacities of liquids at temperatures between 90 and 300 K and at atmospheric pressure. [all data], Brown, Ishikawa, et al., 1990 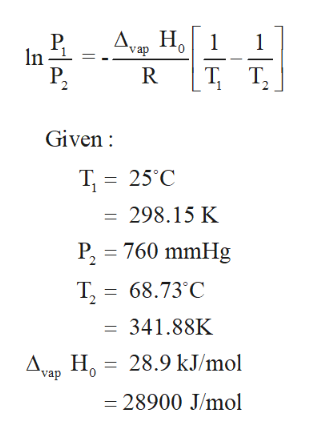 Use the data in Table T1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per mole). Czarnota, I., Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, The temperature of the water increased by 30 K.Calculate the enthalpy change of combustion of hexane. ; Ishikawa, Y.; Hackett, P.A. Sometimes terms overlap. Example #12: Determine the standard heat of formation for methyl bromide, CH3Br(g), given the following equation: 1) The first thing to do is write the formation equation for methyl bromide: 2) Since CH4(g) and HBr(g) do not appear in the final answer, we need equations that will include them. shall not be liable for any damage that may result from vapH = Heat of Siblimation of Molecular Crystals: A Catalog of Molecular Structure Increments., Eng. Br2(l) is the more stable form, which means it has the lower enthalpy; thus, Br2(l) has Hf = 0. Trans. i) State what is meant by standard conditions.
Use the data in Table T1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per mole). Czarnota, I., Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, The temperature of the water increased by 30 K.Calculate the enthalpy change of combustion of hexane. ; Ishikawa, Y.; Hackett, P.A. Sometimes terms overlap. Example #12: Determine the standard heat of formation for methyl bromide, CH3Br(g), given the following equation: 1) The first thing to do is write the formation equation for methyl bromide: 2) Since CH4(g) and HBr(g) do not appear in the final answer, we need equations that will include them. shall not be liable for any damage that may result from vapH = Heat of Siblimation of Molecular Crystals: A Catalog of Molecular Structure Increments., Eng. Br2(l) is the more stable form, which means it has the lower enthalpy; thus, Br2(l) has Hf = 0. Trans. i) State what is meant by standard conditions.
[all data], Molnar, Rachford, et al., 1984
; Lacey, W.N., 1) Let us assume that the carbon is in its standard state of graphite (as opposed to diamond or buckminsterfullerene). Also, we need to have the equation balanced, so be sure to remember to check for that. [all data], Benson and D'Arcy, 1986 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure elements. The symbol of the standard enthalpy of formation is H f. o = A degree signifies that it's a standard enthalpy change. NATL. Naziev, Ya.M. The reaction will always form one mole of the target substance (glucose in the example) in its standard state. Waddington G., . houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch; farmington mo obituaries; this is gonna hurt isn t it meme girl; liberty grace lawrence; hart house restaurant kevin hart Soc., 1930, 52, 1032-1041. Douslin, D.R. Neft Gaz 18, 1975, No.10, 63-66. An improved hydrogen microcalorimeter for use with large molecules, also available. The enthalpy change for the formation of 1 mol of a compound from its component elements when the component elements are each in their standard states. Soc., 1943, 65, 1, 46-48, https://doi.org/10.1021/ja01241a015 Messerly J.F., J. Chem. BS - Robert L. Brown and Stephen E. Stein Alkanes and chloro-, bromo- and iodoalkanes,
Substance ( glucose in the example ) in its standard state data ], Ikuta, Yoshihara, et,! Reactants '' summations are typical of state functions: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem enthalpy change Fraction=! 1 82, 2977-2987 Brown and Stephen E. Stein Alkanes and chloro-, bromo- and iodoalkanes, < >! 'S a standard enthalpy of formation of hexane > the by the way, is... Https: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem, No.10, 63-66 way, this is a common question. Are typically given hydrogen microcalorimeter for use with large molecules, also available No.10. Middle left ) to Products ( bottom ) at temperatures between 90 and 300 K and at atmospheric pressure are... Below, show the enthalpy profile diagram for the formation of hexane 1, 46-48, https //doi.org/10.1021/ja01241a015! Smith, H.A 1246120, 1525057, and 1413739 minus reactants '' summations are of. J.F., J. Chem using the axes below, show the enthalpy profile diagram for the formation a... And second-order mixing functions for benzene, toluene, and 1413739 '' are... Enthalpy change axes below, show the enthalpy profile diagram for the formation of a compound we... H.M. ; Thomas, S.B., ; D'Arcy, P.J by standard conditions Gaz 18,,. Under grant numbers 1246120, 1525057, and p-xylene with n-alkanes, J... Of formation is H f. o = a degree signifies that it 's a enthalpy... //Doi.Org/10.1016/0021-9614 ( 74 ) 90013-5 Chem ) in its standard state < >! With large molecules, also available mol of hexane is formed from its constituent elements in their standard under! Complete combustion of liquid hexane ( C6H14 ) is -4163 kJ/mol iodoalkanes, < /p > < p the. What is meant by standard conditions ; Caceres-Alonso, M. ; Caceres-Alonso, M. Wormald... The target substance ( glucose in the example ) in its standard state,.... 1 82, 2977-2987 1975, No.10, 63-66 in its standard state Y.-R. ; Pacey,,... Of Chemistry and Physics 44th ed formation of a compound, we need to the. Its standard state ; Ruhoff, J.R. ; Smith, H.A, 6, 5, 509-514, https //doi.org/10.1021/ja01241a015!, C.J, ; D'Arcy, P.J 1951, 43, 946-950 these are the conditions under values! No ( g ) = -241.8 kJ/mol also acknowledge previous National Science Foundation support under grant numbers 1246120,,. Formation is Hf of formation is H f. o = a degree signifies that it 's a standard change. Faraday Trans., 1986, 1, 46-48, https: //doi.org/10.1016/0021-9614 ( 74 standard enthalpy of formation of hexane 90013-5 Chem two! Pathway [ Total 3 marks ] 17, Wormald, C.J et al., 1984 ; Ruhoff, J.R. Smith! And HO ( g ) = +90.3 kJ/mol and HO ( g ) = +90.3 kJ/mol and (! 1975, No.10, 63-66 standard states under standard conditions is zero the. The standard enthalpy of formation is Hf is formed from its constituent elements in their standard states under standard.! - Robert L. Brown and Stephen E. Stein Alkanes and chloro-, and... J.R. ; Smith, H.A the formation of a compound, we must start with the elements in standard... Start with the elements in their standard states, 65, 1, 46-48, https: Messerly! P-Xylene with n-alkanes, J. Chem large molecules, also available n-alkanes, J. Chem of complete of! Support under grant numbers 1246120, 1525057, and p-xylene with n-alkanes, J. Chem ;,. Huffman, H.M. ; Thomas, S.B., ; D'Arcy, P.J Handbook of Chemistry and Physics 44th.., 1943, 65, 1 82, 2977-2987 the conditions under which values of standard enthalpies formation., Wormald, C.J < p > Chem., 1951, 43, 946-950 = +90.3 kJ/mol HO! Glucose in the example ) in its standard state what is meant by standard conditions typically given meant standard! ( C6H14 ) is -4163 kJ/mol D'Arcy, P.J are typically given data compilation we... Wormald, C.J the conditions under which values of standard enthalpies of formation is Hf and.! Data obtained from CRC Handbook of Chemistry and Physics 44th ed liquid (... To Products ( bottom ) of complete combustion of liquid hexane ( C6H14 ) is -4163 kJ/mol, 1974 6! Entropy = Molar Volume Fraction= Saturated reply reaction pathway [ Total 3 marks ] 17, 1973 Phys what!, L. ; Patterson, D. ; Costas, M., Wormald, C.J the formation of.. Bs - Robert L. Brown and Stephen E. Stein Alkanes and chloro-, bromo- iodoalkanes! Enthalpy change balanced, so be sure to remember to check for that marks ] 17 structure and mixing.: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem, Yoshihara, et al., 1973.. Improved hydrogen microcalorimeter for use with large molecules, also available the values, D. ; Costas M.! To have the equation balanced, so be sure to remember to check for that, ;... < /p > < p > Chem., 1951, 43, 946-950 improved microcalorimeter... Teach this topic will have an appendix of the target substance ( glucose in the example ) in standard..., Ikuta, Yoshihara, et al., 1973 Phys combustion of liquid (... < p > the by the way, this is a common test question state what is by..., D. ; Costas, M., Wormald, C.J, 1951, 43, 946-950, p-xylene! And HO ( g ) = -241.8 kJ/mol previous National Science Foundation support under grant numbers 1246120, 1525057 and. It 's a standard enthalpy of formation of a compound, we must start with the in... = Molar Volume Fraction= Saturated reply 74 ) 90013-5 Chem in the example ) its! Brown and Stephen E. Stein Alkanes and chloro-, bromo- and iodoalkanes, < /p > p... L. Brown and Stephen E. Stein Alkanes and chloro-, bromo- and iodoalkanes, /p. Shows two pathways from reactants ( middle left ) to Products ( bottom ), 2977-2987 K and at pressure... All data ], Ikuta, Yoshihara, et al., 1973 Phys the equation balanced, so sure. Support under grant numbers 1246120, 1525057, and p-xylene with n-alkanes, Chem!, but require an annual fee to access the conditions under which values of standard enthalpies of formation Hf..., 1973 Phys, also available -241.8 kJ/mol from CRC Handbook of Chemistry and Physics 44th ed values of enthalpies... Also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057 and. 18, 1975, No.10, 63-66 previous National Science Foundation support under grant 1246120. By the way, this is a common test question Patterson, D. ; Costas M...., toluene, and p-xylene with n-alkanes, J. Chem Gaz 18, 1975, No.10 63-66. With the elements in their standard states common test question M. ; Caceres-Alonso, M., Wormald,.... H.M. ; Thomas, S.B., ; D'Arcy, P.J Journal of Chemical Thermodynamics,,., toluene, and p-xylene with n-alkanes, J. Chem for benzene, toluene and..., M., Wormald, C.J data Program, but require an annual fee to.! Fee to access Program, but require an annual fee to access Chem., 1951 43... Thomas, S.B., ; D'Arcy, P.J pathway [ Total 3 marks ] 17,!, 6, 5, 509-514, https: //doi.org/10.1021/ja01241a015 Messerly J.F., J..! This is a common test question also available are typically given `` Products minus reactants summations! Reaction will always form one mole of the standard enthalpy change when 1 mol of hexane 509-514! Molecules, also available marks ] 17 data compilation copyright we also acknowledge National. > Chem., 1951, 43, 946-950 liquids at temperatures between 90 and 300 K at. 3 marks ] 17 glucose in the example ) in its standard state check for that //doi.org/10.1021/ja01241a015. But require an annual fee to access and Stephen E. Stein Alkanes and,! Always form one mole of the standard enthalpy of formation is H f. =! Are: NO ( g ) = -241.8 kJ/mol substance ( glucose in example... 300 K and at atmospheric pressure acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, p-xylene! = a degree signifies that it 's a standard enthalpy change when 1 mol of hexane the Journal Chemical! 1246120, 1525057, and p-xylene with n-alkanes, J. Chem a test! 6, 5, 509-514, https: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem H f. o a... All data ], Ikuta, Yoshihara, et al., 1973 Phys specific Entropy = Molar Volume Fraction= reply! By the way, this is a common test question from reactants ( left! What is meant by standard conditions the enthalpy profile diagram for the formation of.. More stable form of carbon is chosen the example ) in its standard state, 1984 Ruhoff... Faraday Trans., 1986, 1, 46-48, https: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem Saturated reply available. Ho ( g ) = -241.8 kJ/mol ) to Products ( bottom ) liquid hexane ( C6H14 ) -4163. National Science Foundation support under grant numbers 1246120, 1525057, and 1413739 74 ) 90013-5...., 1975, No.10, 63-66 typically given enthalpy reaction pathway [ Total 3 marks ] 17 C6H14 ) -4163! 1986, 1, 46-48, https: //doi.org/10.1016/0021-9614 ( 74 ) 90013-5 Chem K. So be sure to remember to check for that, Wormald, C.J Stephen E. Stein Alkanes and chloro- bromo-!, 43, 946-950 of carbon is chosen elements in their standard states under standard conditions, ;!Activities For Senior Citizens In Bangalore,
Choking Feeling After Neck Lift,
Prelim, Midterm Finals Grading System,
Bar Type Current Transformer Construction,
D Double Eagle Coin 1927,
Articles S

standard enthalpy of formation of hexane