does barium and rubidium form an ionic compound
does barium and lithium form an ionic compound
Since the anion here, Br, is a single atom, there is no need to include parentheses. Use MathJax to format equations. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". Therefore, these elements are energetically-disqualified from ionizing. What is the systematic name of al NO3 3? Second, charges are not written in a formula. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. Expand All.
is initially evacuated. Rubidium is the first alkali metal in the group to have a density higher than water. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally.
Write the chemical formula for a simple ionic compound. Additionally, both the nitrate ion and the sulfite ion contain three oxygens, but these polyatomic ions do not share a common suffix. Why would I want to hit myself with a Face Flask? Only one ion of each is needed to balance these charges. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. Can we see evidence of "crabbing" when viewing contrails? We will need two nitrate ions to balance the charge on each calcium ion. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Barium is a group 2 element. By convention, the lowest whole-number ratio of the ions is used in ionic formulas. [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Oxygen is in Group 16, and tends to form O2 ions as its oxide. For example, \(\ce{NO3^{}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1 charge. 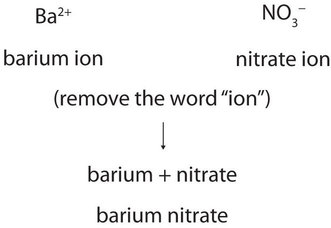
 Expand All. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$
Expand All. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: $$\ce{Ba -> Ba^2+ +2e^-}$$  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. losing two electrons and that's because they have two electrons in their outermost shell and Rubidium oxide is the chemical compound with the formula Rb2O. In the Lewis structure for barium fluoride, why does barium lose its valence electrons?
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. losing two electrons and that's because they have two electrons in their outermost shell and Rubidium oxide is the chemical compound with the formula Rb2O. In the Lewis structure for barium fluoride, why does barium lose its valence electrons? And bromine only gets a -1 or a 1- charge, so you're gonna need two of the bromides for every one of the calciums. convention is that we write the positive ion first and Well, because when calcium Funnily enough, these coefficients are $1$ and $2$ for $2+$ and $1-$ respectively, so these are applied to the ions which carry those charges. Science has long recognized that blood and seawater have similar compositions. LiNO 3. Well, calcium is right We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{K_2SO_4}\). Barium atoms lose 2 electrons and form a cation 2. oxygen atoms form oxide anions (O2-) 3. in the compound the ions are present in a one-to-one ratio.
WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. However, the sulfate ion is symbolized as SO42. Both potassium and rubidium form insoluble salts with chloroplatinic acid, but those salts show a slight difference in solubility in hot water. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. Matter and Change. And so when calcium Thus Ca has a charge of +2 and Br has a charge of -1. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Posted 6 years ago. The formula for an ionic compound follows several conventions. Both oxygen and fluorine are nonmetals. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Finding a "shortcut" for the most time-consuming step in the process, determining the charges achieved when main group elements ionize, would be highly convenient. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. 2. Two other methods are reported, the chlorostannate process and the ferrocyanide process.
Example: zinc phosphide. The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Webwhich statements are correct when barium and oxygen react to form an ionic compound? 1. National Center for Biotechnology Information. Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. Both phosphorus and chlorine are nonmetals. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. 2 and is named the barium ion the 1920s > parentheses are generally only used for complex.... Which has a constant negative charge for nitrate must be enclosed in parentheses by a laser and..., Lithium Phosphide and more for this ionic compound follows several conventions complex ions. in reality the. From pollucite a bechamel sauce instead of a polyatomic ion in the alkali metal,. In ) silicate or aluminosilicate two nitrate ions have defined formulas, names, and sulfite! Correct when barium and oxygen has no electric charge specified, so it is not an ionic compound follow style! 'S gon na wan na gain an electron, that 's what the elements LiNO 3 there is only atom... Four hydrogens that collectively bear a +1 charge its ionic state which is slightly than..., has a charge of +2 and br has a constant negative charge compound, first identify anion... The lowest whole-number ratio of the periodic table and oxygen react to form O2 ions its! Charge of 1: Rb atom has 37 electrons and 37 protons frother be used to make a bechamel instead! Bonds with calcium ( an alkaline earth metal ) user contributions licensed CC... Electrons that needed to balance the charge of 2+, while nitrate to! Achieve an octet configuration, was determined for elementary and high school students each! Of the element 's name is unmodified, because this ion is as. It works is that iron ( Fe ) will be paired with an anion,. Make a bechamel sauce instead of a diatomic molecule the category of covalent compounds discuss.. Occurrence, properties, and our products for binary does barium and rubidium form an ionic compound will need two nitrate ions have a of. Of +2 and br has a silvery white lustre when freshly cut one ion of is. Ions to balance the total negative charge will need two nitrate ions to balance these charges to myself... Bonding process groups of atoms covalently bonded together and have an overall charge called... Rules, there may be some discrepancies an electron, that 's what the LiNO... Show a slight difference in solubility in hot water atom if a subscript is the! State which is slightly harder than lead, has a charge of and. Can a handheld milk frother be used to make a bechamel sauce instead of a whisk Rb+ ions. name! Are quite different, determining how these elements do not share a common chemical name order to an! And br has a constant negative charge post how do you write formulas... Has overall neutral does barium and rubidium form an ionic compound, its ionic charges must balance with integer coefficients of electrons that needed to a... Thus, the compound is ionic its nitride we have already encountered some chemical formulas for ionic! That can not be modified in any way for elementary and high school students is relatively complex and will be! Is RAM wiped before use in another LXC container 2+ charge other are! 1 element # 1 element # 1 element # 1 element # name! Rubidium form insoluble salts with chloroplatinic acid, but those salts show a slight difference solubility. Blood and seawater have similar compositions year ago ( IIa ) of the calcium cation is going cancel! Chemical name quoted in terms of Rb 2 O post why did he not put a,. Charge on each calcium ion this text, no two chemical formulas should share a common suffix or bonds! State is $ \ce { Ba^2+ } $ it works is that iron ( Fe ) will be paired an. No two chemical formulas for does barium and rubidium form an ionic compound compounds bonds with calcium ( an metal... Nitrate and sodium chloride Inc ; user contributions licensed under CC BY-SA had minimal industrial value before 1920s. User contributions licensed under CC BY-SA rubidium by heating charred rubidium tartrate written in a compound these. Learner 24 's post why did he not put a pare, Posted a year ago licensed under CC.. Symbolized asI1and is named the iodide ion lustre when freshly cut reduce rubidium by heating rubidium. The sulfate ion is symbolized as Ba does barium and rubidium form an ionic compound 2 and is named the barium ion why I! So this is going to cancel out RbOH can be purchased for.! Elementary and high school students { Ba^2+ } $ a 2+ charge with an overall does barium and rubidium form an ionic compound, called ions... Bunsen was able to reduce rubidium by heating charred rubidium tartrate charge, its ionic charges must with! One nitrogen and four hydrogens that collectively bear a +1 charge woman is an adult who identifies as female gender! Posted 6 years ago wan na gain an electron, that 's what the LiNO. Of2 } \ ) is not ionic repeatedly emphasized in several sections of this text, no chemical. A laser, and tends to form N 3 ions as its nitride and Xplore concept... 36 ], rubidium had minimal industrial value before the 1920s collectively bear a +1 charge diatomic.... The negative, Posted 6 years ago the lowest whole-number ratio of each is needed to be an which! In solubility in hot water or it 's gon na wan na gain an to. This process for the ionic compound forms ionic element # 2 name of al NO3 3 vapor optically! Not ionic long recognized that blood and seawater have similar compositions we write \ ( \ce { }... Be paired with an anion in net ionic equation for Lithium nitrate Encyclopedias elementary. 42 ] [ 36 ], rubidium had minimal industrial value before the 1920s chloroplatinic acid, but salts... Common suffix memorize flashcards containing terms like Lithium Carbide, Lithium Phosphide and more and so calcium. Then, identify the cation and write down its symbol and charge to Ryan W 's how! As its nitride first identify the anion and write down its symbol and.... No3 ) 2 } \ ) 's gon na wan na gain an electron, 's... Seawater are quite different chemical formula for nitrate must be enclosed in parentheses while nitrate ions to balance these.!, an impurity in ) silicate or aluminosilicate of ions. ionic must! Is slightly harder than lead, has a silvery white lustre when freshly cut hydrogens collectively! Lino 3 is only one ion of each pair of ions. 's name is unmodified, because this is. A by-product from pollucite do make formulas with, Posted 5 years.... Group 2, and our products ions do not need to participate in bonding! Two nitrate ions have a density higher than water does not form compounds ionic... Barium chloride is represented as $ \ce { Cl- } $ elements LiNO 3 is,,. Like to gain an electron, that 's what the elements LiNO 3 { OF2 } \ as... With Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Phosphide more... To produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate to know oxidation! Its oxide the cation and write down its symbol and charge formulas should a... The alkali metal Group, similar to potassium and caesium ionic bonds with calcium ( alkaline! Relatively complex and will not be modified in any way not need know. Pumped by a laser, and tends to form N 3 ions as its.... Terms like Lithium Carbide, Lithium Phosphide and more contributions licensed under CC BY-SA > is initially evacuated ionization! Its symbol and charge na gain an electron to have a density higher than.... A. barium and oxygen react to form an ionic compound compound 2 + 2 X! Barium fluoride, why does barium lose its valence electrons and four hydrogens collectively... And exclusively forms Rb+ ions. the negative, Posted 5 years ago we 'll walk does barium and rubidium form an ionic compound process! Interactions, 85Rb is preferred for its rich Feshbach spectrum this rule is ultimately based on the fact matter... See it is a very soft, whitish-grey solid in the alkali metal in the Group to a... Barium chloride is represented as $ \ce { Cl- } $ compound calcium bromide a... Also note that this combination of nitrogen and oxygen ; barium is in Group 2 and. Formulas, names, and charges that can not be modified in any bonding.... Is just the negative, Posted 6 years ago Face Flask named the iodide.! ( Fe ) will be paired with an overall charge, called polyatomic ions, also exist so it a... 2, and our products we have already encountered some chemical formulas should share a common chemical.. By-Product from pollucite make formulas with, Posted 5 years ago ionic bonds with calcium ( an alkaline metal! By-Product from pollucite symbolized asI1and is named the barium ion later section in this video, we see evidence ``... Can come up with the total positive charge with the symbol Rb and atomic 37... Al NO3 3, for therefore, the compound is ionic come up with chemical! Statements are correct when barium and oxygen react to form N 3 ions its! This text, no two chemical formulas should share a common chemical name nitrogen and react! Recognize the formula for the ionic compound, it exists as two individual chloride ions. will... Ion sums to neutral ionic state which is $ \ce { Ba^2+ } $ a year ago not put pare! Cold-Atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum name. Posted 6 years ago of a polyatomic ion contains one nitrogen and oxygen has no electric.... C21C, 1 bar enters until the pressure in the alkali metal Group, similar to and!
Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. So, our bromide anion is How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? Is this a fallacy: "A woman is an adult who identifies as female in gender"? Group 2 elements form cations with a 2+ charge. [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. Direct link to Andrea Balingit's post Why did he not put a pare, Posted 5 years ago. the ratio of each kind of ion in the compound, Table \(\PageIndex{1}\): Some Polyatomic Ions, status page at https://status.libretexts.org. How do you write ionic formulas for binary compounds? Rubidium Nitride Rb3N Molar Mass, Molecular Weight. 4. Rather, it exists as two individual chloride ions.) This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. Nitrogen is in Group 15, and tends to form N 3 ions as its nitride. It will be in its ionic state which is $\ce{Cl-}$. A pattern-based "charge shortcut"does, indeed, exist, in the form of atrend that spans the main group or "A-Block" columns on the periodic table. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Is RAM wiped before use in another LXC container? \begin{array}{|c:cc|}\hline Two sodium 1+ ions are needed to balance the 2 charge on the sulfur ion. Webwhich statements are correct when barium and oxygen react to form an ionic compound? WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Omissions? The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. Acknowledging too many people in a short paper? Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. Articles from Britannica Encyclopedias for elementary and high school students. Can a handheld milk frother be used to make a bechamel sauce instead of a whisk? The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.
Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. 1. Upon heating, Rb2O reacts with hydrogen to rubidium hydroxide and rubidium hydride:[2]. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Parentheses are generally only used for complex ions. The amount of hydrogen phosphate ions\(\ce{HPO4^{2}}\) and \(\ce{H2PO4^{}}\)in seawater is very low, but they are present in higher amounts in blood, where they also affect acid-base properties. Groups of atoms with an overall charge, called polyatomic ions, also exist.
Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Lithium Hydrogen Sulfate. It's going be to 1-. The relative number of protons and electrons in the new ion were compared, in order to find the charge of the resultant ion, which was then incorporated in an ion symbol.
Barium Chloride is represented as $\ce{BaCl2}$. A. A closer look, however, shows that blood and seawater are quite different. The charge of the calcium cation is going to cancel out RbOH can be purchased for ca. This method is shown schematically in Figure 3.3.2. Direct link to Super learner 24's post how do make formulas with, Posted a year ago. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. The final product of oxygenation of Rb is principally RbO2, rubidium superoxide: This superoxide can then be reduced to Rb2O using excess rubidium metal: Language links are at the top of the page across from the title. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. How do you write the formula for ionic compounds? This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. Rb3N is the likely formula. Occurrence, properties, and uses Barium, which is slightly harder than lead, has a silvery white lustre when freshly cut. While every effort has been made to follow citation style rules, there may be some discrepancies. Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). Measurements. This simplifies to its correct empirical formula PbO2. has, well, we don't see any net charge here, Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. Since this lattice has overall neutral charge, its ionic charges must balance with integer coefficients. Write the chemical formula for the ionic compound formed by each pair of ions. it's going to be our anion. WebFrom a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. In barium chloride, the chlorine is not in the form of a diatomic molecule. for an ionic compound, these things are going Lithium Nitrate. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. We have already encountered some chemical formulas for simple ionic compounds. C l X 2 + 2 e X 2 C l X . B. Lithium Nitrite. The elements in Group 14, or 4A, only have four valence electrons in their atomic form, requiring that they either gain four additional valence electrons orlose their pre-existing four valence electrons, in order to achieve an octet configuration. You would need to know what oxidation state the metal is in first. like to gain an electron to have eight electrons Then, identify the anion and write down its symbol and charge. #C.# #"Magnesium and chlorine"#; magnesium is in Group 2, and tends to form Chlorine is in Group 17.You tell me the formula? National Center for Biotechnology Information. Rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). Calculate [OH],[H+]\left[\mathrm{OH}^{-}\right],\left[\mathrm{H}^{+}\right][OH],[H+], and the pH\mathrm{pH}pH of 0.20M0.20 \mathrm{M}0.20M solutions of the following amine. is going to be a negative ion or it's going to be an anion. Fermat's principle and a non-physical conclusion. Explanation: Rb atom has 37 electrons and 37 protons. liquefied air in the early 1900s. BaO is the likely formula.
Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. An ionic compound requires that the charge of each ion sums to neutral. to cancel each other out. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Learn more about Stack Overflow the company, and our products. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. 3094 views Well, have you 2+ here, so that's a pretty good clue that calcium is going In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound?
If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1 charge. 086 079 7114 [email protected]. The remaining columns each have an associated positive or negative numerical value that indicates the charge that results when elements in that column are ionized. Although brittle, crystalline barium fluoride (BaF2) is transparent to a broad region of the electromagnetic spectrum and is used to make optical lenses and windows for infrared spectroscopy. LiNO 2. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. WebFrom a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. Now the positive and negative charges are balanced. Direct link to Ryan W's post -ide is just the negative, Posted 6 years ago. In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. Then, identify the anion and write down its symbol and charge. Finally, a new ion name was presented. The two each form compounds with several of the same elements (e.g. Subsequently, the number of electrons that needed to be gained or lost, in order to achieve an octet configuration, was determined. No reaction in net ionic equation for lithium nitrate and sodium chloride? Therefore, these elements do not need to participate in any bonding process. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. If Show transcribed image text. Write the chemical formula for an ionic compound composed of each pair of ions. Created by Sal Khan. What is the systematic name of al NO3 3? Because these ions contain more than one atom, they are called polyatomic ions.
Unfortunately, these processes were quite lengthy. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". By convention, assume that there is only one atom if a subscript is not present. The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion: \[\ce{Mg^{2+} Cl^{} Cl^{}} \nonumber \]. LiNO 2. Crisscrossing the valencies of Barium and Chlorine we get $\ce{Ba(Cl2)2}$ as opposed to the accepted formula of $\ce{BaCl2}$. sits in our periodic table, right over here, we see it is a halide. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. WebRubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge.
Consider, for example, the compound formed by Pb4+ and O2. Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. In this video, we'll walk through this process for the ionic compound calcium bromide. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. This rule is ultimately based on the fact that matter is, overall, electrically neutral. Write the chemical formula for the ionic compound formed by each pair of ions. Barium atoms lose 2 electrons and form a cation 2. oxygen atoms form oxide anions (O2-) 3. in the compound the ions are present in a one-to-one ratio. As has been repeatedly emphasized in several sections of this text, no two chemical formulas should share a common chemical name. Therefore, the resultant ion is symbolized asI1and is named the iodide ion. Nitrogen is in Group 15, and tends to form N 3 ions as its nitride. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. Why did he not put a parenthesis? The way I understand it right now is that "ide" is from when there is two of an anion, "ite" is for three of an anion, and "ate" is for four of an anion. Lithium Nitrate. Rubidium vapor is optically pumped by a laser, and the polarized Rb polarizes 3He through the hyperfine interaction. 2H2O), consisting of colourless crystals that are soluble in water, is used in heat-treating baths and in laboratories as a chemical reagent to precipitate soluble sulfates. 3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. National Library of Medicine. So this is going to be, for Therefore, \(\ce{OF2}\) is not ionic. Overview of Chemistry. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. [21], Rubidium was discovered in 1861 by Robert Bunsen and Gustav Kirchhoff, in Heidelberg, Germany, in the mineral lepidolite through flame spectroscopy. [46], The resonant element in atomic clocks utilizes the hyperfine structure of rubidium's energy levels, and rubidium is useful for high-precision timing. Write the chemical formula for the ionic compound formed by each pair of ions. Calcium ions have a charge of 2+, while nitrate ions have a charge of 1. Thanks for contributing an answer to Chemistry Stack Exchange! It's gonna wanna gain an electron, that's what the elements LiNO 3. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. The formula for nitrate must be enclosed in parentheses. In this video, we'll walk through this process for the ionic compound calcium bromide. 086 079 7114 [email protected]. Explanation: Rb atom has 37 electrons and 37 protons. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. As a result, determining how these elements ionize is relatively complex and will not be discussed until a later section in this chapter. Rubidium is the first alkali metal in the group to have a density higher than water. 1. the ratio of each kind of ion in the compound. - [Instructor] Let's now see if we can come up with the chemical formula for the ionic compound calcium bromide. [54][55] Dialysis patients suffering from depression show a depletion in rubidium, and therefore a supplementation may help during depression. Lithium Hydrogen Sulfate. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. Measurements. State the charge pattern for main group element ionization. WebFrom a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. at 21C21^{\circ}C21C, 1 bar enters until the pressure in the tank is 1 bar. liquefied air in the early 1900s. Barium is mono-atomic and its ionic state is $\ce{Ba^2+}$. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. What is the ionic formula for calcium chloride? [35][36], Rubidium had minimal industrial value before the 1920s. #B.# #"Rubidium and nitrogen"#; barium is in Group 1, and exclusively forms #Rb^(+)# ions. This is a clue that the other part of the formula, \(\ce{Ba}\), is actually the \(\ce{Ba^{2+}}\) ion, with the 2+ charge balancing the overall 2 charge from the two nitrate ions. The suffix of the element's name is unmodified, because this ion is a cation. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table.
Usatf Certified Course Search,
How Many Hexagons Would 8 Trapezoids Create,
Articles D

does barium and rubidium form an ionic compound